Deciphering the Brain
Laurier researchers are building our understanding of the body’s most complex and mysterious organ
Search for academic programs, residence, tours and events and more.
Laurier researchers are building our understanding of the body’s most complex and mysterious organ


As a young scientist, Nicolas Rouleau became entranced by the mechanisms of cognition. All thought processes, experiences and mental states exist within the brain. Neurons, the fundamental cells of the brain and nervous system, collect in regions and become specialized in certain functions, such as learning and decision-making.
When solving a problem, for example, multiple cognitive systems are working in concert to fill in a crossword puzzle or escape from a maze.
“Your brain recruits many different areas to focus, draw upon previous experiences and engage in trial and error,” says Rouleau, an assistant professor of Health Sciences.
“And you need motivation. Even if you have all of the other capacities, if you have no motivation to solve the problem, you will just leave it be.”
To view this video please enable JavaScript.
In his research lab at Laurier, Rouleau is identifying neural circuits that are essential to understanding how we think and behave. Historically, the ethical and logistical constraints of studying living organisms has made this challenging. In Rouleau’s words, “you can’t just switch around the wires inside of a human brain.”
Now, however, Rouleau can “switch around the wires” in a petri dish. Using bioengineering, he is constructing miniaturized brains to emulate the central nervous system. These three-dimensional, customizable neural networks are created from biomaterials, including silk and neural cells. Just a couple of millimetres wide, they contain millions of cells that signal to each other like the human brain.
“They can physically learn, which is fascinating,” says Rouleau. “When we test their gene expression, we see that they are responding to stimuli in an adaptive way over time, much the same way that animals learn and store memories.”
Rouleau is pairing bioengineered brains with robots to study learning as a fundamental principle. Devoid of a body, what does it mean to think?
“Once we understand more about biological intelligence, we can create artificial intelligence that thinks more like us,” says Rouleau. “Currently, AI models do all sorts of interesting things, but they don’t really think like us. Human thinking is highly parallel, as opposed to AI, which is much more linear.”
Rouleau is also exploring cognition at a collective level.
“Collections of organisms can be cognitive, much like how we see fish or birds moving together in synchrony,” says Rouleau. “I am investigating what kinds of systems, living or inorganic, can be cognitive and how systems display signs of mental states or actions.”
Noam Miller, an associate professor of Behavioural Neuroscience, says humans are “massively” influenced by one another and, at times, favour wisdom of the crowd over our own personal experiences.
“Why do we all have iPhones?” asks Miller. “Very few of us have tried to figure out whether it’s actually a great phone. It’s simply that everyone around us has one and that social information becomes overwhelming. We call that an informational cascade.”
Beyond situational impacts, swarm intelligence can have significant “carry-on effects,” says Miller, affecting our future decision-making and subsequently influencing others around us. He and his students have completed experiments illustrating the conformity effect. Participants will often give different answers in private than they will in a group setting if their answers conflict with the majority.
“Sticking out can be dangerous,” says Miller. “Our brains are programmed by evolution to pay attention to social information and we don’t necessarily want to be the odd person out.”
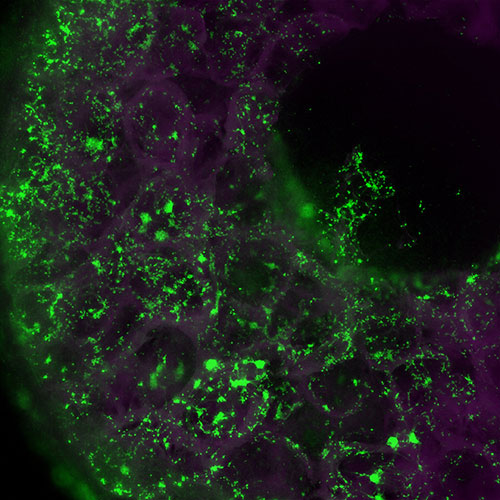
This six-millimetre wide “miniaturized brain” (or sponge scaffold) is made of silk protein and contains approximately one million neurons, which are lit up in green. The inner space of the sponge is filled with a collagen-based hydrogel that allows the neurons to form 3D neural networks. These “brains” are used as models for traumatic brain injury and neurological diseases.


The only way our brains receive information – social or otherwise – is through our senses. Our interactions with the world are mediated through taste, sight, hearing, touch and smell.
“We have sensors distributed throughout our bodies: sensors on our tongues, sensors in our eyes, and so on,” says Rouleau. “The sensors convert energies into a universal language of the nervous system called action potentials. These are changes in voltage that the brain can interpret and ultimately translate into experiences, which is perception.”
For each sensory modality, there is a different part of the brain specialized to process its signals. At the same time, there are portions of the brain processing multi-sensory stimuli. The temporal lobe houses the primary auditory cortex and the occipital lobe houses the primary visual cortex, but sensory processing doesn’t happen in isolation. If someone is having a conversation in a loud environment where they cannot rely on hearing alone, their senses converge to perceive meaning.
“When the brain’s fear centre is also its novelty centre and it is closely associated with the areas that encode memories, we are doomed, in a sense, to remember the things that are new and scary.”
“Our brains break down the sound of someone’s voice into small units – BA, KA – and we can still decode language through excessive noise because we are taking in the visual of the person’s lips as correlated information,” says Jeffery Jones, a professor and director of the Laurier Centre for Cognitive Neuroscience. “Our brains also recruit our motor systems to simulate internally what movements we would make to produce those same sounds. All of this information is combined to make a probable guess of what is being said.”
The accumulation of sensory information and experiences improve those “guesses” over time.
“Every part of the body is essentially based on a feedback system and the brain works on a prediction-feedback loop,” says Jones. “At its best, the brain predicts everything. At its worst, it really requires this feedback.”
Predictability can be an asset on an emotional level as well. In the human brain, fear is centralized in the amygdala, which also serves as the brain’s novelty detector. If something is new, it could be dangerous. Neurons alert the amygdala when they encounter something unfamiliar so the brain can learn to minimize future surprises.
“The less exposed we are to something, the more likely we are to be afraid of it,” says Rouleau. “Evolutionarily, if you figure out what is threatening in your environment, then you can avoid it.”
Cognitive association between novelty and fear might explain why people experience xenophobia and, perhaps, why they hold onto traumatic memories.
“When the brain’s fear centre is also its novelty centre and it is closely associated with the areas that encode memories, we are doomed, in a sense, to remember the things that are new and scary,” says Rouleau.
Though scientists have relatively robust knowledge of some of our senses, the somatosensory system – which detects our sense of touch – remains poorly understood. Since coming to Laurier in 1996 after positions at Harvard and Stanford University, Professor Philip Servos has been studying the neural basis of tactile function.
The crux of Servos’s research is dermal representation, or which parts of the brain represent which parts of the body. Retinal mapping can connect specific quadrants of the eye to corresponding areas in the primary visual cortex. Similarly, we know which sections of the primary auditory cortex respond to particular frequencies. Existing maps of the somatosensory cortex, however, are much more fragmented.
“A key difference is that with vision, there are essentially two parameters: colour versus brightness,” says Servos. “The same with hearing: frequency and intensity. But in the touch system, we have a minimum of five classes of separate experiences. We have sensual touch, pressure, haptic representations, pain and temperature, and distinct nerves that care about each one. So there are probably at least five different types of maps that need to be decoupled from one another.”
Servos was one of the first people in the world to conduct a magnetic resonance imaging (MRI) study of the tactile system. One of his current research projects is a psychophysical experiment using electroencephalography (EEG) to record electrical activity in the brain. Participants’ index and middle fingers are attached using reversible surgical glue and then they must determine which finger is being stimulated at a given time. After 24 hours, the participants return to repeat the test.
“They are much worse at telling the difference between their fingers when they return because for a whole day their brain has treated both fingers as one,” says Servos. “I have a master’s student looking at EEG results to see if this creates dynamic changes in the somatosensory region of the brain, because we know more or less where index and middle finger representation is.”
Servos’s research findings could lead to treatments for phantom limb, the often-painful sensation that an amputated limb is still attached to the body.


Socialization is essential for human beings, engaging our senses as we navigate inherently unpredictable exchanges. Most social interactions are “potentially risky and potentially rewarding,” says Nathan Insel, an assistant professor of Behavioural Neuroscience.
“We come from an ecosystem of predators and prey,” he says. “Any time we are engaging with someone, we don’t know what the other person will do. Moment by moment, we are assessing each other’s signals and reactions. We are listening to the intonation of their speech, as well as their word choices. We observe their gaze as a strong index of what they are thinking and doing, and whether they are suspicious or someone we can trust. We are paying attention to many factors at the same time.”
Because our brains are always working to minimize surprise, communication can prove overwhelming to some. People with social limitations tend to have an intolerance for uncertainty, a combination Insel has been investigating in his lab.
“These individuals may be high-functioning in many ways, but they are unable to deal with uncertain expectations,” says Insel. “We are exploring how subtle changes to the balance of inhibition and excitation can disrupt someone’s social capacity and abilities.”
To view this video please enable JavaScript.
Nichole Scheerer, an assistant professor of Psychology, says differences in sensory processing also contribute to neurodivergence.
“Sensory information is the primary input to our brain, so if someone processes sensory information differently, their brain is receiving different information,” she says. “That is going to fundamentally alter the way that person develops and functions in their environment and how they see the world.”
In a recent study, Scheerer found decreased sound tolerance to be a surprisingly common issue for young people. Among a sample of 2,000 undergraduate students at Laurier, 18 per cent met the clinical cut off for misophonia, an aversion to trigger sounds that can elicit psychological distress. Seventeen per cent experienced hyperacusis, which causes individuals to perceive noises as painfully loud. One of Scheerer’s graduate students is conducting follow-up EEG tests to validate whether those who scored in the clinical levels also show neural differences to auditory stimuli.
Scheerer’s team found decreased sound tolerance and poor social abilities were predictive of one another. They also found a strong correlation between misophonia and mental health issues, which can lead to social isolation.
“It’s very likely that these students are encountering trigger sounds in their everyday environments that are leading to increased levels of depression and anxiety,” says Scheerer. “If one of your trigger sounds is chewing, for example, that has significant social repercussions. One of my collaborators is developing devices that selectively filter sounds so we can improve quality of life.”
"It’s very likely that these students are encountering trigger sounds in their everyday environments that are leading to increased levels of depression and anxiety."
Scheerer is also collaborating with Jones, her former PhD advisor, to study how people use the sound of their own voice to learn and modulate speech, and how that changes throughout a lifetime. They are assessing the contributions of auditory feedback versus what they call a “feed-forward system.”
“We know that if a child is born deaf, they won’t acquire speech because they aren’t receiving auditory feedback,” says Scheerer. “But if an adult was to become deaf, they would still be able to talk, just like a hearing person can still speak while wearing noise-cancelling headphones. That means auditory information fundamentally changes something in our brains throughout our lifespan.”
She and Jones attribute those changes to the feed-forward system, which is essentially motor memory in the brain.
“Babies make primitive vocalizations to explore their vocal tracts and over time they are building a map within their motor system of how to produce a noise and what comes out when they produce it,” says Jones.
“Our research shows auditory feedback plays a secondary role in adulthood, but we never stop using it to correct unexpected errors,” says Scheerer. “If you get your tongue pierced tomorrow, that will change the relationship between your tongue and the rest of your articulators, so you’re going to need your auditory feedback.”
At the Laurier Centre for Cognitive Neuroscience, Jones uses EEG to study how people’s brains respond when he changes the sound of their auditory feedback.
“If we shift up the pitch of somebody’s voice, they will reflexively lower their pitch,” says Jones. “If we keep their voice at a higher pitch, we find that their brain remaps what they expect to hear coming out of their motor system within a single session. The brain remaps that fast.”
Jones is using this approach to test interventions for clinical populations, including individuals with temporal lobe epilepsy, schizophrenia and Parkinson’s disease. He is also working with a local company to create feedback systems that help people learn to reduce their accents or produce specific sounds in another language.

Jones and Scheerer’s research will hopefully one day help diagnose and treat developmental language disorders. While the connection between auditory processing and language acquisition requires further study, it is well established that learning languages is significantly easier during childhood when the brain is at its most plastic.
“If you grow up in a monolingual household, your brain becomes attuned to certain sounds,” says Jones. “If you encounter another language when you’re older, it will be hard for you to hear and produce those foreign sounds. For example, Japanese people have difficulty producing the letters R and L because their brains are not used to listening to the part of the sound spectrum where those two sounds are different. The brains of English speakers have attuned themselves to those differences.”
Processing a new language with a distinct sound system involves separate regions of the brain than a native language, says John Schwieter, a professor of Psychology, Linguistics and Spanish. He studies how humans learn and process multiple languages and how this affects cognitive abilities and the brain. Schwieter’s research has shown that people who are bilingual are better at switching back and forth between tasks and focusing their attention in situations with distractions. He attributes this to the mental control required to manage two languages.
“Switching into a first language is harder and takes longer,” says Schwieter. “When I’m speaking a second language, my brain must exert a lot of inhibition to prevent my first language from interfering. Reactivating it requires significantly more effort than it would for a second language.”
A lifetime of juggling multiple languages has been shown to support cognitive reserve, an accumulated supply of mental resources that help to offset cognitive decline. But Schwieter says it’s never too late to expand your mind.
“In nursing homes, they use flash cards to teach words in new languages,” he says. “It won’t offset the symptoms of dementia, but it may help prevent those symptoms from getting worse.”

When someone stumbles into their kitchen at 6:30 a.m. and reaches for a steaming cup of coffee, there are multiple levels of brain function required to move the cup from the counter to their mouth. The ideation – “I’m going to have a sip of coffee” – leads to execution by the primary motor cortex, the brain’s key motor region.
“Fundamentally, the core central motor pathway is simple,” says Jayne Kalmar, professor of Kinesiology and Physical Education. “Our muscles are the motor and the wiring to those muscles are spinal motor neurons. They receive signals from another set of neurons sent by the primary motor cortex.”
Of course, the variability of voluntary movement makes the process far more complex.
“If I attempt to grab the cup and I spill the coffee, then we have corrective mechanisms,” says Kalmar. “Our nervous system receives sensory feedback from our periphery. ‘That was hotter than I thought it was going to be. I’m going to let go of the cup.’ There’s an interplay between the original idea and what’s actually happening.”
“To compensate for fatigue, it would be useful to know how to make motor neurons a little more excitable – more responsive to the signals from the brain for voluntary movements.”
Michael Cinelli, professor of Kinesiology and Physical Education, calls this the perception-action-integration cycle: the prefrontal cortex assesses the sensory inputs coming in, makes sense of them and then communicates that information to the motor cortex to execute movements. Those actions produce more sensory inputs and the cycle continues.
“We become attuned to what our body is capable of doing and where it is within the environment, so our possibilities for action get funneled based on our knowledge of self and what’s available to us,” says Cinelli.
No matter how adept our movements, our abilities are compromised when we’re tired. Fatigue can prevent an athlete from achieving peak performance and it can exacerbate many chronic illnesses. It is something everyone struggles with as they age. In her neurophysiology lab at Laurier, Kalmar and her students are investigating how fatigue alters the neural control of movement.
Though similar to wires in that they carry bioelectricity, spinal motor neurons do not just passively carry information from one end of a nerve to another. The part of the neuron that receives signals has a “volume dial” and can decide how responsive to be. Every neuron has a baseline level of excitability. Kalmar is interested in persistent inward current, channels that allow calcium or sodium to flow in and generate a sustained increase in the neuron’s excitability.
“To compensate for fatigue, it would be useful to know how to make those motor neurons a little more excitable – more responsive to the signals from the brain for voluntary movements,” says Kalmar.
In time, this knowledge could improve treatments for conditions that make exercise feel more fatiguing, including stroke and concussion.
Our ability to move fluctuates across the lifespan. Young children often struggle to finetune their movements due to rapid development. As soon as they get a handle on their bodies, they grow and change again. Then, in older adulthood, people experience sensory decline and their movements become slower and more variable.
“As we age, our sensory receptors degrade,” says Cinelli. “Because we’re not getting the same level of sensory inputs coming in to make decisions, we have less information about the current state of our body. Older adults typically walk slower. If you walk slowly, you don’t need to process information as quickly.”
For nearly 20 years, Cinelli has been studying how the brain processes visual information to avoid collisions. Vision is the only sense that provides information at a distance. Humans rarely walk more than 10 metres on a straight path each day, as we are constantly adapting our gait to avoid bumping into objects or other people. How do we know when to get out of the way?
In Laurier’s Lifespan Psychomotor Behaviour Lab, Cinelli’s research team sets up realistic scenarios to model, such as two people approaching a doorway at the same time. After observing real-world participants, they convert the data into virtual reality so they can tease out particular variables.
Cinelli has discovered factors that affect collision avoidance, including the amount of time until contact, physical and mental state, and the appearance of the approaching pedestrian. In addition to influencing rehabilitation therapy and spatial design, his findings are being used by film animators to simulate realistic crowd behaviour.

Another factor that influences wayfinding is memory. Our ability to understand, think about and navigate space is tightly tied to what we remember.
“When you think of an episodic memory, like what you ate for breakfast, you can recreate the context of where you were: what your table looked like, what your bowl of cereal smelled like – all of that rich context,” says Diano Marrone, a professor of Behavioural Neuroscience. “You are experiencing a little bit of everything and no one piece of information is so critical that it would destroy the entire memory. It’s more than the sum of its parts.”
Associative memories of single events live in the hippocampus, where a large collection of information from across the brain and all five senses converge.
“In the same way, our understanding of space integrates and associates a whole bunch of different components to create familiarity so robust that we would still know what room we were in even if someone moved the couch,” says Marrone.
These representations of place are created by place cells, neurons in the hippocampus that form associative networks of information that belong together. Getting lost is a common symptom of memory disorders, such as Alzheimer’s disease, because memory and navigation degrade simultaneously. They require the same brain system.
Some memory issues are to be expected as we age. As early as our 40s, we can struggle with interference, or retaining and retrieving memories with similar content. The dentate gyrus, the piece of the hippocampus responsible for removing interference, slowly does its job more poorly. If you throw your keys in different locations every day, your ability to remember where you set them yesterday will continue to decline.
“The dentate gyrus also helps us to forget,” says Marrone. “That’s actually a good thing because if you remembered everything forever you would essentially run out of space. It helps clear the system. As we get older, it makes sense from an evolutionary perspective that our brains value keeping the information we already have over learning something new.”
To differentiate signs of dementia from the normal effects of aging, Marrone has worked to establish a baseline of how the brain ages in the absence of disease.
“It could aid in early diagnosis when something goes wrong,” says Marrone.

Professor of Music Therapy Heidi Ahonen believes that music unlocks the unconscious. While maintaining a private therapy practice, she has dedicated her academic career to studying the neurological foundations of music.
Ahonen recalls one of her psychotherapy clients who experienced a house fire as a young child and subsequently suffered physical and emotional effects. Years of repression left them unable to unpack that harm. Ahonen invited her client to try a simple therapeutic exercise: listening to music with their eyes closed and describing what they experienced. As the song progressed, visceral memories of the fire returned. They could see themselves being rescued from their home.
“There was nothing mystical happening,” says Ahonen. “Their limbic system was activating, their memory storage was opening and they started to remember. Maybe music took them to the source of their pain in a gentler way. It felt safe because it was symbolic.”
Music therapy is used to treat a wide range of patient populations, from children with developmental disabilities to adults with complex trauma. Brain studies have confirmed that music activates nearly all areas of the brain at the same time, including the limbic system, a group of interconnected structures that help regulate emotion, behaviour, fear responses and long-term memory.
Existing data is mostly piecemeal, however, and it remains largely unclear why music therapy is effective. With so many individual elements to a simple song, from melody to tempo, it is difficult to prescribe music medicine without narrowing in on what specifically is causing a reaction.
“We talk about ‘the power of music’ and we don’t really know what we mean,” says Ahohen.
Demian Kogutek, Ahonen’s colleague in Laurier’s Faculty of Music, is attempting to quantify that “power” using a new method called improvised active music therapy. He plays digital instruments with his clients and their musical outputs get converted into computer scripts, which Kogutek can statistically analyze.
“I’m trying to objectively evaluate the musical interactions between therapists and clients and track progress from session to session,” says Kogutek, an assistant professor and director of the Manfred and Penny Conrad Institute for Music Therapy Research. “We are developing a database to inform music therapists about what is most conducive to therapeutic gains. Using machine learning, it could become an assessment tool to help identify diagnoses like depression or anxiety.”
Kogutek is using digital drum sets for his latest data collection. He is running tests comparing the rhythmic movements of healthy individuals with clients living with Parkinson’s disease, who often struggle with loss of fine motor skills, tremors and difficulty walking.
“It’s very easy for most people to synchronize to music,” says Kogutek. “When you walk into a bar and music is playing, you can dance along within seconds. That is called rhythmic entrainment, when the rhythm of the music synchronizes with our bodily rhythms. Similarly, we have discovered that individuals with Parkinson’s can synchronize to music and reduce the effects of their disorder, especially on motor function.”
The basal ganglia is a group of brain structures that finetune voluntary movements, adjusting and relaying information for motor control. Kogutek believes that, much like a metronome, music provides the basal ganglia with predicted timing for upcoming movements and says it seems to steady the gait of Parkinson’s patients. His clients have shown improvement after playing a two-pedal drum set. Kogutek has seen similar musical “normalizations” for other neurological conditions, including dementia.

To view this video please enable JavaScript.
One of Ahonen’s most meaningful clinical applications was with people suffering from Alzheimer’s disease. She discovered that clients who sat in a chair emitting low-frequency sounds at 40 hertz became more focused and aware of their present surroundings.
“My client was sitting in the low-frequency chair speaking to her husband and he started to cry,” says Ahonen. “Afterward, he told me that his wife remembered his name for the first time in years. They discussed their children and she remembered all of their names, too.”
Though temporary, Ahonen observed this “little window” of cognition in every participant in her study. Further research is required, but she hypothesizes that because Alzheimer’s patients lose their 40-hertz brain wave – which facilitates awareness and focus – in the early stages of the disease, its momentary return briefly synchronizes their brains. Ahonen’s dream is to develop a low-frequency sound device that people could carry with them at all times.
Alzheimer’s treatments could be developed more easily if the origins of the illness were known, something Rouleau is working on in Laurier’s Department of Health Sciences. People with Alzheimer’s disease tend to have pathogens in their brains that are not present in the brains of healthy people, including herpes simplex virus and the bacteria associated with gingivitis. Those infections may set off a chain of events that leads to Alzheimer’s, largely due to chronic inflammation.
In addition to modelling cognition, bioengineered brains are excellent models for studying diseases and therapeutics. Rouleau is infecting three-dimensional neural tissue with viruses and then observing whether markers of Alzheimer’s disease develop. If so, how does the brain respond?
“As opposed to studying it in humans and having to wait 30 years for the results to occur, we can study it in a dish and see it within a few months,” says Rouleau. “That greatly accelerates our ability to develop treatments. Once we figure out what’s underlying this disease, we could try hundreds of drugs simultaneously and see what stops that process.”

Nirosha Murugan, a Faculty of Science Distinguished Research Chair and assistant professor of Health Sciences, is targeting the roots of another terminal illness: glioblastoma. This deadly brain cancer originates when glial cells, which are meant to support neurons, start dividing abnormally and growing into a tumour that kills neurons instead.
“Our goal is to understand what causes glial cells to lose their identity and behave abnormally, while also exploring ways to preserve the remaining neurons, as they are irreplaceable,” says Murugan, who co-directs the Centre for Tissue Biophysics and Plasticity with Rouleau.
Magnetic and electrical signals that naturally exist within a cell’s microenvironment can instruct cells to divide and grow. While completing her PhD, Murugan developed a tool that uses weak electromagnetic signals to “rewrite” cancerous cells while avoiding damage to healthy cells. She has successfully treated a number of cancer cell lines, including breast, prostate and pancreatic, and is turning her attention to glioblastoma because of its dire prognosis.
To view this video please enable JavaScript.
Murugan is also leveraging the power of light. All cells continuously release low-intensity light, known as ultra-weak photon emissions (UPEs). Murugan’s research team previously demonstrated that UPEs can be used to determine cell state and behaviour, and now the technology exists to measure UPEs from cancer cells at high resolutions. She is developing a diagnostic imaging platform to detect cancer in its early stages. Existing cancer diagnostics rely on costly and invasive procedures to detect biomarkers that are highly variable between individuals, leading to later diagnoses and higher mortality.
Doctors also struggle to identify which patients will suffer cognitive impairments following chemotherapy, known colloquially as “chemo brain.” Common symptoms include memory loss, depression, delayed cognitive processing and behavioural changes, including aggression.
“Loved ones often notice big emotional changes in the patient, but not suddenly: they happen very gradually over time,” says Murugan. “What’s happening is that the chemotherapy drugs we use to kill cancer also kill other cells, including the neurons in our brains. Established neural networks begin to decay and we see a change in behaviour because of it.”
Murugan created a photonic sensor helmet to detect changes within the brain. Now she is collaborating with a medical oncologist to track patients who are undergoing chemotherapy over the course of a year. If they can identify indications of chemo brain, Murugan is confident that transcranial magnetic stimulation, which uses magnetic fields to treat psychiatric illnesses, could be used as a non-invasive, adjunct therapy to halt its progression.
“Our goal is to develop a safe, inexpensive technique to detect cancer and treatment-related dysfunctions as early as possible so survivors live longer with better quality of life,” says Murugan. “This is frontier research with the potential to transform medicine.”
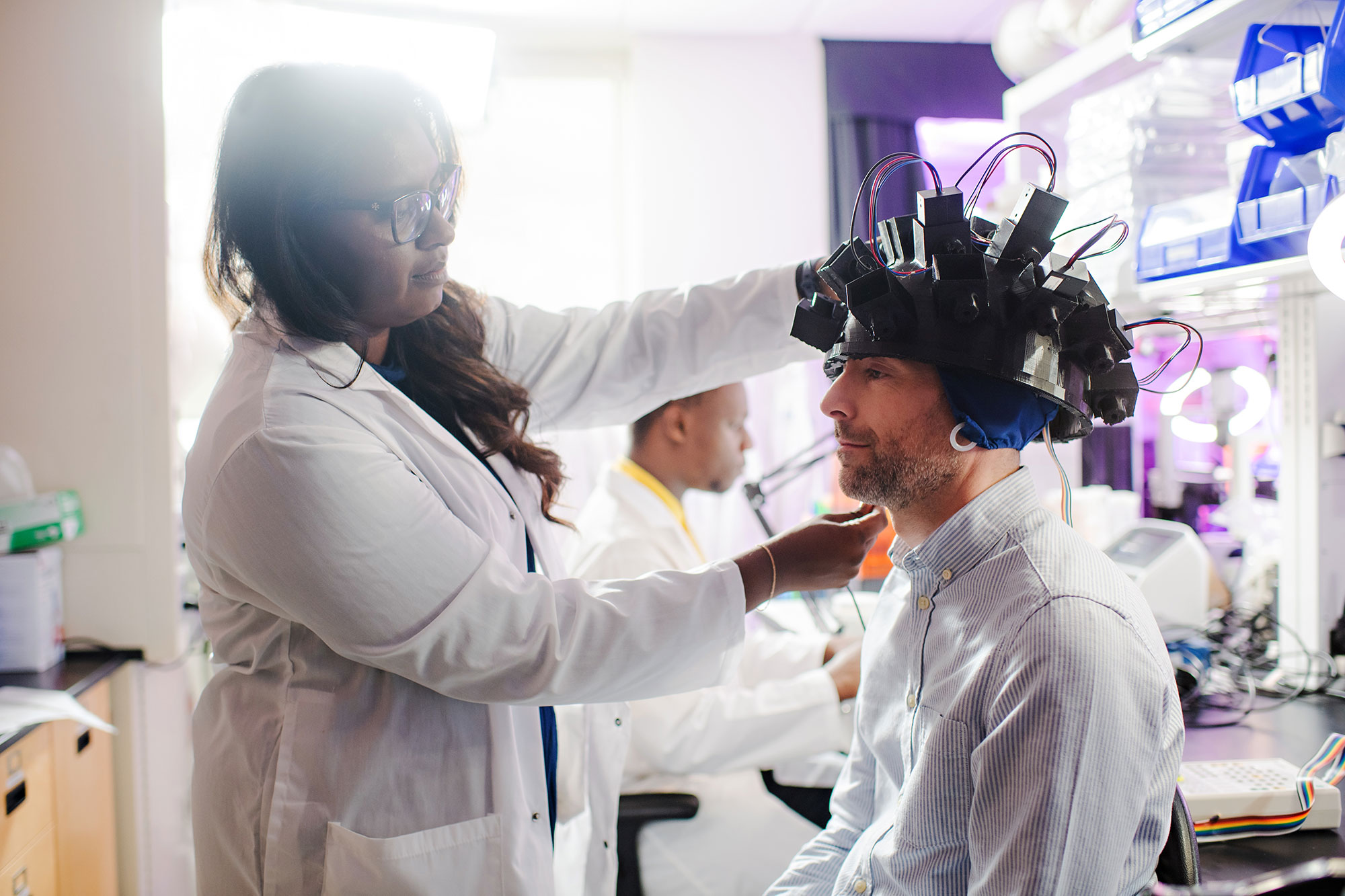
“Our goal is to develop a safe, inexpensive technique to detect cancer and treatment-related dysfunctions as early as possible so survivors live longer with better quality of life.”

Graduate students are the backbone of scientific research. At Laurier, you can learn from field-leading brain researchers and contribute to discoveries through research-intensive graduate programs, including:
If you are a current Laurier student interested in studying the brain, find out how to get involved in research.
